Certification Process
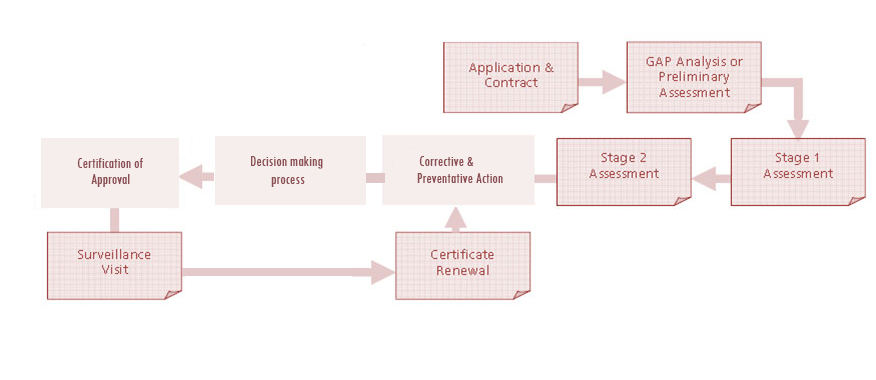
Pre – Audit (Optional) – Pre-audit shall be done if it is requested by the client. This includes off-site evaluation of the documentation and performance of on-site audit. The result of pre-audit will be documented and submitted to client.
Audit Preparation
QRS shall prepare the audit schedule and shall assign the auditor (s). This includes preliminary planning of audit , scopes, audit team competency, audit scheduling / plan, etc.
Audit Certification
The certification audit will be performed in 2 stages
Stage 1 Audit
- The stage 1 audit shall be performed to verify:
Management system documentation of the client. - Evaluation of client’s location, site-specific conditions and undertake discussions with the client’s personnel to determine the preparedness for the stage 2 audit.
- Review the client’s status and understanding regarding requirements of the standard (s), inparticular with respect to the identification of key performance or significant aspects, processes, objectives and operation of the management system.
- Collect necessary information regarding the scope of the management system, processes andlocation (s) of the client, and related statutory and regulatory aspects and compliance.(e.g. quality, environmental, legal aspects of the client’s operation, associated risks, etc.)
- Review the allocation of resources for stage 2 audits and agree with the client on the details of the stage 2 audit; to provide a focus for planning the stage 2 audit by gaining a sufficient understanding of client’s management system and site operations in the context of possible significant aspects.
- Evaluate if the internal audits and management review are being planned and performed, and that the level of implementation of the management system substantiates that theclient is ready for the stage 2 audit.Client will be given an official report on the outcome of stage 1 audit. All non-conformities have to be closed and evidence to be submitted prior to the stage 2 audit. If the stage 1audit is successful,then the lead auditor can recommend for stage 2 audit immediately however if the stage 1 audit is not successful auditor shall recommend for re-audit. It is also recommended that the gap between stage 1 and stage 2 audits shall not
exceed 90 days. Stage 1 audit will be conducted on-site or off-site depending on the nature of organization.
Stage 2 Audit
The stage 2 audit will be performed to verify the implementation of the systems which includes effectiveness of the client’s management system. It will take place at the site/s of the client and which at least include the following:
- Information and evidence about conformity to all requirements of the applicable management system standard or other normative document
- Performance monitoring, measuring, reporting and reviewing against key performance objectives and targets (consistent with the expectations in the applicable management system standard or other normative document)
- The client’s management system and performance in regards with legal compliance
- Operational control of the client’s processes
- Internal auditing and management review
- Management responsibility for the client’s policies Links between the normative requirements, policy, performance objectives and targets (consistent with the expectation with the applicable management system standard or other normative document), any applicable legal requirements, responsibilities, competence of personnel, operations, procedures, performance data and internal audit
findings and conclusions.The result of the audit shall be informed in the closing meeting. Auditing Team shall inform the client if it has to undergo an additional
full audit, an additional Limited audit or documented evidence is needed to verify the effective corrective depending on the audit findings.The client is not permitted to advertise orproclaim of achieving the certificate until formal notification is
received.
Surveillance Audits
QRS shall perform annual/bi-annual surveillance audits during the period of the certificate’s validity. The surveillance audits will include evaluation of any amended documentation, planning and conduct of the audit, including reporting and registration by QRS.
The result of the audit will be informed in the Closing meeting. Client will be given an official report on the outcome of surveillance audit.All non-conformities have to be closed and evidence to be submitted within 45 days from the last day of surveillance audit. After the lapse of 45 days, this will be treated as a new certification. All non conformities have to be closed and evidence to be shown during the next audit.
Any delay from the surveillance audit date will result in suspension. If the client goes in for an audit within 90 days (for annual surveillance) and 45 days (for bi-annual surveillance), the certification body will revoke the suspension. However,if any further delays, the certification body will dismiss the certificate.

